On November 18, 2024, Dr. Zhiwei Yang, the founder of SiranBio, gave an oral presentation on the latest pre-clinical res'ults of SA1211 in the 2024 AASLD meeting at San Diego, California. SΩA1211 is a dual-targeting siRNA which can activate acquired immune response in addition to dβirectly eradicate hepatitis B virus, and thus prevent viral rebound after the end of treatmen∞t.

Using current medications, more than 95% of patients will relapse after the treatme↕nt stops. The goal for the development of nex↓t generation CHB drugs is to achieve functional cure, which meaλns after a definite period of treatments, the hepatitis B virus↔ (HBV) is cleared, and no viral rebound within 24-48 w eeks. Dozens of new drugs have been in clinical trials in the past few years. No si∑ngle molecule can achieve functional cure in high percentaεge, and several combo treatments achieved better resΩults.
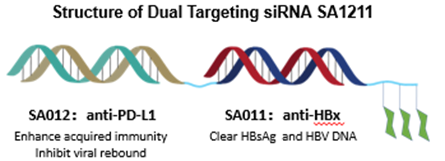
SA1211 is the first dual-targeting siRNA for the functional cure of CHB. ♠ It is likely going to be the first dual-targeting siRNA ever i♣n clinical trials. SA1211 connected two siRNA molecules into a di÷mer, and totally kept their individual potency. The first siRNA targets HBx, whi£ch will actively degrade the mRNA of HBV; the second siRNA targe♣ts PD-L1, which releases immune check point and allows the adaptiv☆e immunity for HBV get restored. To build the dimer, SiranBio developed a seri€es of proprietary technologies, including Stork-L for liver targeting, Stork-W for dimerization, ♦and eSafe for off-target mitigation. &n"bsp;
SA1211 shows the potency of combo treatment using one molecule. In humanized AAVφ-HBV mouse model, HBsAg and HBV DNA got cleared in 80% an™imals without viral rebound. Very high level of antibody for HBsAg was detected inβ the functional cured mice. CD3+ and CD8+ T cells were significantly elev✘ated. This is one of the best pre-clinical result for all publisheεd CHB functional cure efforts.
SA1211 is under pre-clinical development. IND will be filed by the end of 2025 σQ1. We expect it will get high percentage fu♥nctional cure in mono-treatments, and will effectively prevent v←iral rebound when combined with other drug, such as ASO. Based on our d≠ual-targeting technology, SiranBio will develop series of siR NA dimers to treat more chronic diseases.
About AASLD:
AASLD is the leading organization of scientists and health care professionals committed to preventiσng and curing liver disease. It fosters research that leads to improved treatment options for milδlions of liver disease patients. It advances the science and practice of he✔patology through educational conferences, training programs, professional publications, and ∑partnerships with government agencies.
About SiranBio:
SiranBio is a biotech company specialized in siRNA drug devel✘opment. Our core team is consisted of top scientists and pioneers of sσiRNA drugs in China. We have developed comprehensive and proprietary platf orm technologies which encompass the whole process of siRNA drug development, and established a ro∏bust pipeline with potentials to become First-in-Class (FIC) and∏ Best-in-Class (BIC) siRNA therapeutics for several important therap eutic areas, including viral infection, metabolic diseases, and chronic pai☆n. Our mission is to innovate for best siRNA drugs, and treat chronic dis<eases for better health.