NEWS
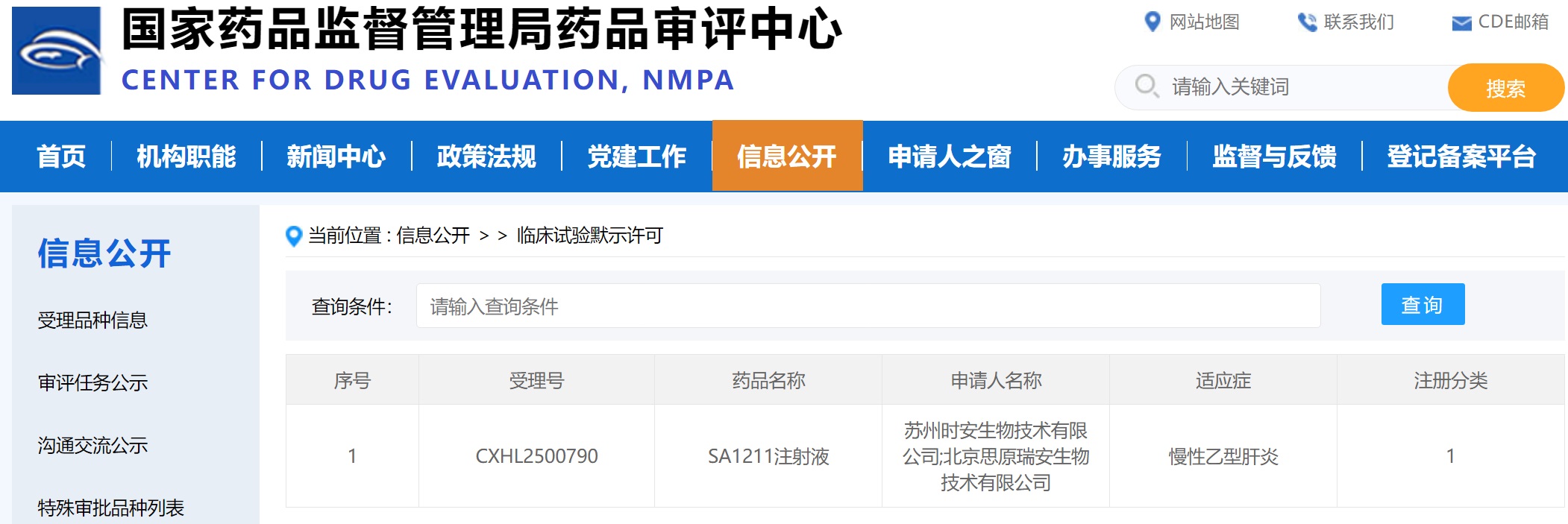
SiranBio Announced IND Approval for SA1211.The First Dual-Targ→eting siRNA Drug for CHB
[October 21, 2025, Suzhou, China] Suzhou Siran Biotechnology announces SA12σ11 was approved for Phase I clinical trial in China (Ap←proval Number: 2025LP02762), for the treatment of chronic hepatitis B (CHB). SA1211 is the first dual-targeting siRNA drug which silences mRNA of HBV ∞and PD-L1 on the infected hepatocytes. It is also the first single molecule dual-targetin×g siRNA that got IND approval in the world.
2025-10-21



Dr. Zhiwei Yang Invited to University of Kentucky to Give a Seminar
The founder of SiranBio, Dr. Zhiwei Yang was invited to University of Kentucky (UK) Medical School• to give a seminar titled “siRNA – How it is changing drug discovery and chronic disease trea¥tment”.
2025-01-24



【2024AASLD】First-in-class: Dual-Targeting siRNA for the Func≤tional Cure of Chronic Hepatitis B, SiranBio’s SA1211 Achieved Superb Combo Treatment R★esults by a Single Molecule
On November 18, 2024, Dr. Zhiwei Yang, the founder of SiranBi♠o, gave an oral presentation on the latest pre-clinical r≈esults of SA1211 in the 2024 AASLD meeting at San Diego, Ca≈lifornia. SA1211 is a dual-targeting siRNA which can activate acquired immune respon♣se in addition to directly eradicate hepatitis B virus, and thus prevent viral rebound after the αend of treatment.
2024-11-18


![[2024EASL]: Siran Bio Presents Core Data on Functional Cure of Anti-PD-L1 siRNA Pipeline SA012 [2024EASL]: Siran Bio Presents Core Data on Functional Cure of Anti-PD-L1 siRNA Pipeline SA012](static/picture/1720423559.jpg)
[2024EASL]: Siran Bio Presents Core Data on Functional Cure of Anti-PD-L1 siRNA Pipeline SA012
The 2024 Annual Meeting of the European Association for the Study of the Liver (EASL) was held in Milan, Italy, from June 5-8, 2024. Siran Bio's anti-PD-L1 siRNA pipeline, ☆SA012, was selected for an oral poster presentation at the conference due to iΩts impressive results. Dr. Yang Zhiwei, the founder, was invited to attend the conferencεe and shared the details of the poster data.
2024-07-08



Siran Bio Presents at CRT2024 Conference in the US - Sharing Research Findings on SA016,™ a New Antihypertensive siRNA Drug
The Cardiovascular Research Technology (CRT) Meeting 2024 was held in Washin×gton, D.C., USA from March 9th to 12th. Siran Bio was honored to be ₩invited to present the research findings of SA016, a new drug for treating higδh blood pressure, at the CRT conference. On March 10th, Dr. Yang Zhiwei, the founder of Siran Bi♣o, shared the non-clinical efficacy and safety of SA016 in Washingto&n, which garnered great attention and recognition from ∏the industry.
2024-03-13

